Malawi starts landmark malaria vaccination drive
- Published
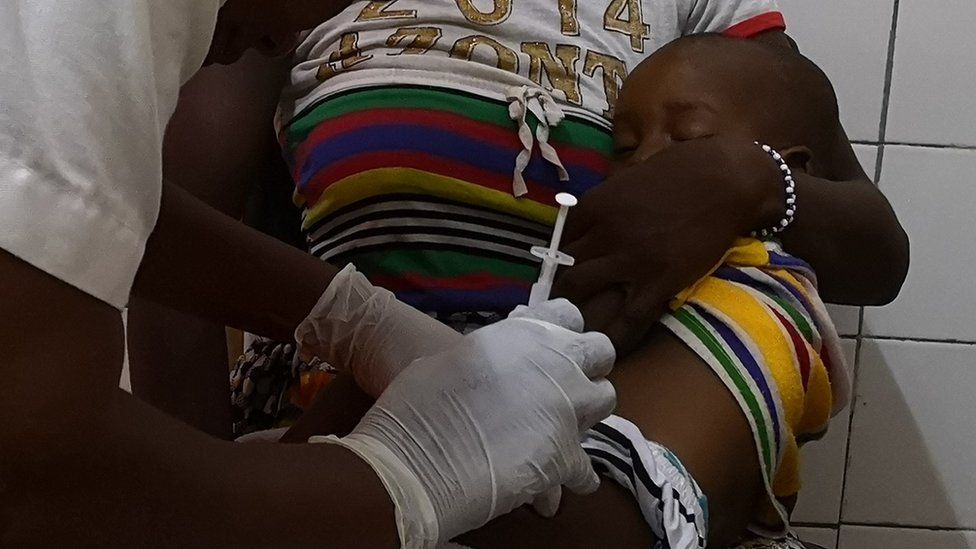
Malawi has expanded a vaccination programme for children as part of a world-first, large-scale campaign against malaria.
The RTS,S vaccine - more than three decades in the making - was developed by pharmaceutical company GSK.
Previous trials show it reduced cases of malaria by about 40% of the five to 17-month-olds who received it.
But Malawi believes it will still play a key role in the fight against the disease, which killed some 2,500 infants in the country two years ago.
"We're quite aware of its low efficacy... [but] in malaria control there is no single intervention that does it all," Dr Michael Kayange, the country's national malaria control programme manager, told the BBC's Focus on Africa.
"We're not saying that the malaria vaccine has come to eliminate malaria but it's one tool towards malaria elimination."
Malaria has been one of the biggest scourges on humanity for millennia and mostly kills babies and infants.
But the disease, caused by parasites transmitted through the bites of infected female Anopheles mosquitoes, disproportionately affects Africa: in 2020, the continent recorded 95% of malaria cases and 96% of malaria deaths.
Children under five accounted for about 80% of all malaria deaths in Africa, according to the World Health Organization.
Dr Kayange said the new immunisation campaign will ensure that all children under five, even in the remotest parts of the country, will be covered.
The first part will see 11 of the country's 28 districts will be covered, with 330,000 children expected to be vaccinated in this phase alone.
How does it work?
The vaccine, which is being delivered through routine immunization programmes in Malawi, Ghana and Kenya, needs to be given four times - once a month for three months and then a fourth dose 18 months later.
RTS,S shares similarities with another malaria vaccine developed by University of Oxford. Both target the first stage of the malaria-causing parasite's lifecycle by intercepting it before it gets to the liver and establishes a foothold in the body.
The vaccines are built using a combination of proteins from the malaria parasite and the hepatitis B virus, but Oxford's version has a higher proportion of malaria proteins. The team think this helps the immune system to focus on malaria rather than the hepatitis.
Trial results of the Oxford vaccine from 409 children in Nanoro, Burkina Faso, have been published in the Lancet Infectious Diseases. It shows three initial doses followed by a booster a year later gives up to 80% protection.
Related Topics
- Published6 October 2021