A drug that’s been described as a transformational miracle for people living with cystic fibrosis (CF) is being made available to younger Albertans living with the progressive disease.

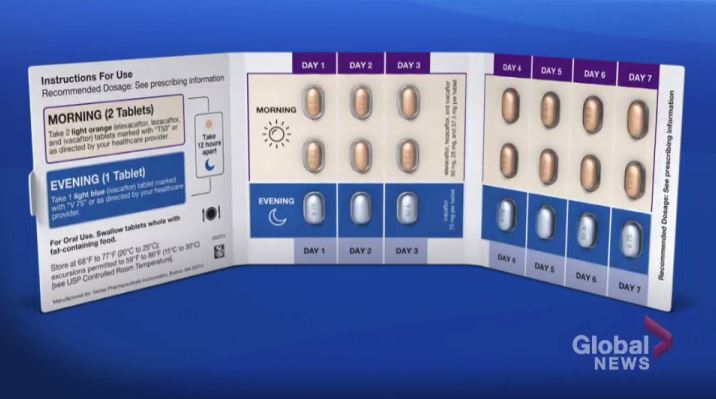
Trikafta is a breakthrough prescription drug: rather than just treating symptoms of CF, Trikafta targets the basic defect from one specific genetic mutation that causes CF.
In 2021, the government announced for Albertans 12 years of age and older, it would start covering Trikafta — which costs about $300,000 a year, per patient.
Alberta was one of the first provinces to do so.
In the summer of 2022, it expanded coverage to kids aged six to 11. On Wednesday, the province announced access has been expanded to children aged two to five, after Health Canada approved the expanded use on Oct. 16.
“This medication has helped many individuals, and now more children can benefit from the improved health and quality of life provided by this treatment,” Health Minister Adriana LaGrange said in a statement.

According to Cystic Fibrosis Canada, approximately 40 young Albertans with the F508 gene meet the medical criteria for Trikafta and are eligible for the medication.
Trikafta could reduce the number of people living with severe lung disease by 60 per cent, and the number of deaths by 15 per cent.
It can also add years of life to those with CF. For example, a child born with the disease could live a decade longer.
“Starting young children with cystic fibrosis on modulator therapy as early as possible could protect their health and prevent significant structural lung damage from occurring — we celebrate this news alongside our CF community in Alberta, who has worked tirelessly for this day,” Cystic Fibrosis Canada president and CEO Kelly Grover said.

CF is a deadly genetic disorder mainly affecting the digestive system and the lungs of children and young adults.
The persistence and ongoing infection in the lungs, with destruction of tissue and loss of lung function, will eventually lead to death in the majority of people with CF.
There is no cure and before treatments began to be discovered, the average life expectancy for someone with CF was 18 years. Now, life expectancy has improved to about 52 years of age as science has advanced.
There are four drugs available in Canada to treat the disease, called cystic fibrosis transmembrane conductance regulators or CFTR modulators. There are Kalydeco, Orkambi, Symdeko and Trikafta.
According to Cystic Fibrosis Canada, there are about 4,300 Canadian children, adolescents, and adults living with the disease.


Comments